-
PDF
- Split View
-
Views
-
Cite
Cite
Chiyoko Sekine, Yuko Moriyama, Akemi Koyanagi, Noriko Koyama, Hideko Ogata, Ko Okumura, Hideo Yagita, Differential regulation of splenic CD8− dendritic cells and marginal zone B cells by Notch ligands, International Immunology, Volume 21, Issue 3, March 2009, Pages 295–301, https://doi.org/10.1093/intimm/dxn148
Close - Share Icon Share
Abstract
The importance of Notch signaling to maintain CD8− dendritic cells (DCs) in the spleen has recently been revealed. However, the ligand responsible for this Notch signaling has not been determined yet. In this study, we demonstrated that blocking of Delta-like (Dll) 1 alone had no significant effect on the maintenance of CD8− DCs while marginal zone (MZ) B cells were significantly reduced in the spleen of mice. On the other hand, blocking of Dll1, Dll4, Jagged1 and Jagged2 significantly decreased CD8− DCs. All these Notch ligands are expressed predominantly in the red pulp of the spleen where CD8− DCs reside. These results indicate a differential regulation of CD8− DCs and MZ B cells by Notch ligands in the spleen.
Introduction
Notch signaling pathways are evolutionarily conserved and play key roles in cell fate determination and differentiation in many tissues during embryonic and postnatal development (1). Four mammalian Notch receptors have been identified, designated as Notch1–Notch4. Interaction of Notch receptors with membrane-bound ligands of the Delta and Jagged families [Delta-like (Dll) 1, Dll3, Dll4, Jagged1 and Jagged2] is critical for Notch signaling. The ligand binding induces γ-secretase-mediated cleavage and translocation of Notch intracellular domain into the nucleus, where it interacts with a DNA-binding protein RBP-J to induce the expression of downstream target genes such as Hes1 and Deltex1 (2).
Recently, a critical role for the Notch signaling has been revealed in the homeostasis of innate immune cells, such as dendritic cells (DCs) and marginal zone (MZ) B cells (3–6). DCs play an important role in the immune system, especially in innate immunity by recognition and capture of invading pathogens. This induces DC maturation to activate adaptive immunity (7). DCs are composed of distinct subsets, such as conventional DCs and plasmacytoid DCs (8). In the spleen, two major subsets of conventional DCs are found, distinguished in mice by the expression of surface marker CD8 (9). CD8+ DCs reside mainly in the T cell zone and are uniquely capable of cross-presenting exogenous antigens via the MHC class I pathway, while CD8− DCs are in the red pulp and MZ and preferentially present exogenous antigens via the MHC class II pathway (9). The CD8− DCs can be also identified by their specific expression of DC inhibitory receptor 2, recognized by the 33D1 mAb (9).
A recent report has shown that splenic CD8− DCs selectively decreased in DC-specific RBP-J conditional knockout (cKO) mice with defective DC-mediated cytokine responses (3). Progenitors of the CD8− DC subset were not affected but CD8− DCs showed increased apoptosis in the spleen of the cKO mice, suggesting that RBP-J regulates the maintenance of committed CD8− DCs by facilitating their survival in the spleen. Messenger RNA of Notch1, 2, 4 and its target genes Hes1 and Deltex1 were expressed in CD8− DCs. This Deltex1 expression in CD8− DCs was completely abolished in the absence of RBP-J, while Notch receptors were expressed (3). Among Notch ligands, Dll1 had been shown to promote DC maturation in vitro (10) and Dll1-positive cells were located in close contact with 33D1+ CD8− DCs in the spleen (3). Thus, it was suggested that Dll1 might be responsible for the Notch signaling that promoted the survival and maintenance of CD8− DCs.
It has been shown that conditional inactivation of Dll1 led to a selective loss of MZ B cells in the spleen (4). Similarly, studies using conditional gene inactivation have shown that the Notch2 signal in B cells is essential for the generation of MZ B cells at the branching point of MZ B and conventional follicular B cells in the spleen (6). In that case, Msx-2-interacting nuclear target protein was shown to be a signal modifier in the cytoplasm (5). Based on these findings, it has been suggested that Notch2 and Dll1 interaction regulates the MZ B cell development in the spleen.
In this study, we have investigated the effect of Dll1 blocking on splenic CD8− DC population to determine whether Dll1 is really involved in the maintenance of CD8− DCs. We have found that Dll1 plays an important role to regulate CD8− DCs but other Notch ligands also participate in it. On the other hand, Dll1 plays a non-redundant role for the development of MZ B cells. These results indicate a differential regulation of CD8− DCs and MZ B cells by Notch ligands.
Materials and methods
Mice
C57BL/6 mice were purchased from Charles River (Tokyo, Japan). All procedures were in accordance with institutional regulations for animal experiments.
Antibodies
Generation and characterization of hamster IgG mAbs specific for mouse Notch1 (HMN1-12), Notch2 (HMN2-35), Notch3 (HMN3-133), Notch4 (HMN4-14), Jagged1 (HMJ1-29), Jagged2 (HMJ2-1), Dll1 (HMD1-5) and Dll4 (HMD4-2) have been described in our recent papers (11, 12). Briefly, flow cytometrical analysis showed that each mAb specifically reacts with CHO cells expressing respective Notch receptor or ligand but not with the other ones. Blocking activities of the anti-ligand mAbs have been verified in vitro or in vivo. Administration of these mAbs does not deplete the cells expressing respective ligand in vivo. FITC-labeled mAbs against mouse CD11c (HL3) and CD21 (7G6), PE-labeled mAb against mouse CD23 (B3B4) and PE-conjugated streptavidin were obtained from eBioscience (San Diego, CA, USA). Biotin-labeled mAb against mouse DC inhibitory receptor 2 (33D1), PerCP-labeled mAb against mouse B220 (RA3-6B2) and non-labeled mAb against mouse CD31 (MEC13.3) were obtained from BD PharMingen (San Jose, CA, USA). Non-labeled mAb against mouse reticular fibroblasts [endoplasmic reticulum (ER)-TR7] was from BMA Biomedicals (Augst, Switzerland). FITC-labeled mAb against mouse F4/80 (CI:A3-1) was from CALTAG (Carlsbad, CA, USA).
Treatment with mAbs
Mice at 6 weeks old were injected intra-peritoneally with 0.25 mg each of the indicated mAbs or control hamster IgG (eBioscience) twice a week for a week (on days 0 and 3). The spleen was removed for flow cytometric and immunohistological analyses on day 7. For the kinetics study, mice were similarly treated once and the spleen was analyzed on indicated days.
Flow cytometry
Spleen was digested with collagenase (Wako, Tokyo, Japan) for 30 min at 37°C, filtered and subjected to red blood cell lysis. Multicolor staining was conducted using combinations of the indicated mAbs. Briefly, spleen cells were first incubated with FcBlock (BD PharMingen) and then with an optimized dilution of biotinylated mAbs. After washing, the cells were incubated with FITC-, PE-, PerCP-labeled mAbs or streptavidin. For apoptosis detection, cells were stained with antibodies, FITC-labeled Annexin V (Biolegend, San Diego, CA, USA) and 7-amino-actinomycin D (BD PharMingen). The cells were analyzed on FACScan or FACScaliber (BD Biosciences) and analyzed with CellQuest (BD Biosciences).
Stimulation of splenocytes
Collagenase-treated splenocytes were stimulated with 3 μg ml−1 imiquimod (Alexis, San Diego, CA, USA), 3 μg ml−1 R848 (Alexis) or 1 μg ml−1 LPS (Sigma, St Louis, MO, USA) in DMEM/10% FCS (Nissui, Tokyo, Japan) for 2 h. Then, add GolgiPlug (BD Biosciences) and incubate for additional 4 h. Cells were stained for surface markers, fixed, permeabilized and stained with PE-conjugated anti-mouse IL-12 (Biolegend).
Immunohistological staining
Tissue samples of spleen were frozen in optimal cutting temperature compound (SAKURA, Tokyo, Japan) and were cut into 3-μm sections. After being air-dried, sections were fixed in acetone, blocked with control hamster IgG or with 3% BSA/PBS for 33D1 staining and stained with biotin-conjugated 33D1, HMD1-5, HMD4-2, HMJ1-29 or HMJ2-1. For detection, Catalyzed Signal Amplification System, peroxidase (Dako Cytomation, Carpinteria, CA, USA) or Tyramide Signal Amplification Kit (PerkinElmer, Walthmam, MA, USA) was used. For staining ER-TR7, CD31 or B220, sections were blocked with 10% normal goat serum and Alexa488-labeled goat anti-rat IgG (Molecular Probes, Carlsbad, CA, USA) was used as secondary antibody. For staining with FITC-labeled mAb to F4/80, sections were blocked with 10% normal rabbit serum and Alexa488-labeled rabbit anti-FITC (Molecular Probes) was used as secondary antibody. Images were acquired on a microscope (Eclipse E800M; Nikon, Tokyo, Japan) equipped with a digital camera (DXM1200; Nikon) and processed using ACT-1 (Nikon). Fluorescent images were acquired on a microscope (Eclipse TE300; Nikon) equipped with a digital camera (C4742-98; Hamamatsu photonics, Hamamatsu, Japan) and processed using AquaCosmos (Hamamatsu photonics).
Results
Expression of Notch receptors and ligands on splenic DCs
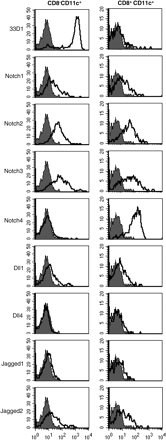
We first examined the expression of Notch receptors and ligands on cell surface of splenic DCs. Flow cytometrical analysis defined 33D1 expression exclusively on the CD8− DC subset (Fig. 1). Notch1, Notch2 and Notch3 were expressed at similar levels on both CD8− and CD8+ subsets. In contrast, Notch4 was highly expressed on CD8+ DCs but not CD8− DCs (Fig. 1). Expression of Notch ligands was also similar between CD8− DCs and CD8+ DCs: Dll1 and Jagged2 were expressed at moderate levels and Jagged1 was expressed at a low level (Fig. 1).
Expression of Notch receptors and ligands on splenic DCs. Expression of Notch receptors and ligands on CD8− and CD8+ sub-populations of CD11c+ splenic DCs were analyzed by flow cytometry. Filled histograms indicate the staining with control Hamster IgG. Open histograms indicate the staining with 33D1, HMN1-12 (Notch1), HMN2-35 (Notch2), HMN3-133 (Notch3), HMN4-14 (Notch4), HMD1-5 (Dll1), HMD4-2 (Dll4), HMJ1-29 (Jagged1) or HMJ2-1 (Jagged2).
Blocking of all Notch ligands, but not Dll1 alone, reduces CD8− DCs in the spleen
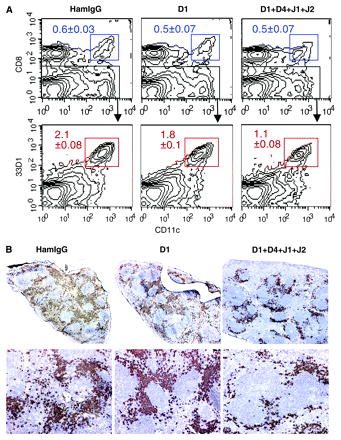
To examine the role of Dll1 in the maintenance of CD8− DCs, mice were treated with anti-Dll1-blocking mAb (HMD1-5) (12) on days 0 and 3, and the spleens were removed on day 7. As shown in Fig. 2(A), the blocking of Dll1 alone had little effect on the population of CD8−33D1+CD11c+ cells (CD8− DCs) or CD8+33D1−CD11c+ cells (CD8+ DCs) in the spleen. No significant change was also observed on days 14 and 28 or with a high dose (1 mg) of HMD1-5 (data not shown). In addition, the percentage of CD8− DCs in total splenocytes of Dll1 cKO mice (4) was not significantly reduced as compared with control mice (data not shown). Then, we additionally blocked Dll4, Jagged1 and Jagged2 by administrating a mixture of mAbs to these molecules (HMD4-2, HMJ1-29 and HMJ2-1, respectively). The combined blocking of Dll1, Dll4, Jagged1 and Jagged2 significantly reduced the CD8− DC compartment to a half of the control hamster IgG treatment (P = 0.000096), while the CD8+ DC compartment was not affected (Fig. 2A). Immunohistochemical analysis showed a substantial decrease of 33D1+ cells in the splenic red pulp of the combination-treated mice (Fig. 2B).
Effect of anti-Dll1 mAb on the maintenance of CD8− DCs. (A) Flow cytometric analysis of CD8+ and CD8− DCs in the spleen of mice treated with control hamster IgG (HamIgG), HMD1-5 (D1) or a mixture of HMD1-5, HMD4-2, HMJ1-29 and HMJ2-1 (D1 + D4 + J1 + J2). CD8− DCs were detected as CD8− gated CD11chigh/33D1+ cells (lower panels). The percentages among whole splenocytes are indicated. Data are represented as the mean ± SD of three mice in each group. (B) Immunohistological analysis of the spleen of mice treated as in (A). Frozen sections were stained with 33D1 mAb in brown. Representatives of three mice in each group. Original magnification: upper panels, ×40; lower panels, ×100.
Relative contribution of each Notch ligand to the maintenance of CD8− DCs and MZ B cells
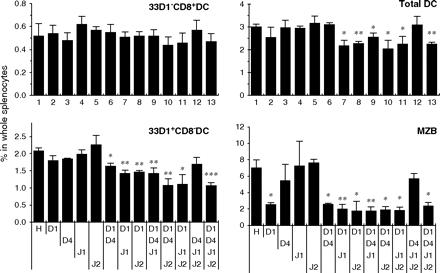
To address whether all these Notch ligands (Dll1, Dll4, Jagged1 and Jagged2) contribute to maintain CD8− DCs, one of these was excluded from the combined blockade. Blocking of Dll4, Jagged1 and Jagged2 did not reduce CD8− DCs significantly, while other blocking combinations of three Notch ligands reduced them significantly, suggesting the importance of Dll1 for the maintenance of CD8− DC subset (Fig. 3). Moreover, combinational blockade of Dll1 with Dll4, Jagged1 or Jagged2 also decreased CD8− DCs though blocking of any single Notch ligand did not (Fig. 3). These results suggest that Dll1 could be important but not only Dll1 but also Dll4, Jagged1 and Jagged2 contribute to the maintenance of CD8− DCs. On the other hand, MZ B cells were decreased exclusively by blockade of Dll1 but not the other Notch ligands (Fig. 3). Notably, the changes of total DCs (CD11c+) in the spleen reflected those of CD8− DCs, while the CD8+ DC compartment was not affected by any of the indicated treatments (Fig. 3).
Effects of anti-Notch ligand mAbs on the maintenance of CD8− DCs. Mice were treated with control hamster IgG (H) or the indicated combination of mAbs (D1, HMD1-5; D4, HMD4-2; J1, HMJ1-29; J2, HMJ2-1). The percentages of CD11chigh/33D1+/CD8− (33D1+CD8− DC), CD11chigh/33D1−/CD8+ (33D1−CD8+ DC) and CD11chigh (total DC) cells among whole splenocytes and the percentage of MZ B cells (B220+/CD21hi/CD23lo/−) among total B cells (B220+) were analyzed by flow cytometry. Data were represented as the mean ± SD of three mice in each group. Similar results were obtained in two independent experiments. Data were analyzed statistically using unpaired Student's t-test. *P < 0.05, **P < 0.01, ***P < 0.001 versus hamster IgG-treated control mice.
Kinetics of splenic CD8− DC reduction
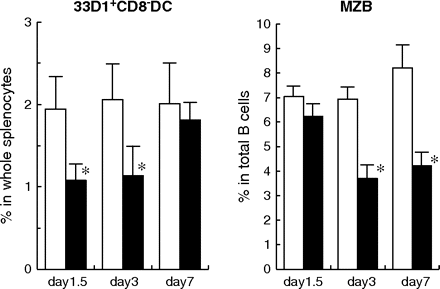
It has been shown that Notch signal-deficient CD8− DCs exhibited increase of apoptosis in the spleen and suggested that Notch signals were required for their survival (3). Actually, the treatment with a mixture of antibodies against all Notch ligands (HMD1-5, HMD4-2, HMJ1-29 and HMJ2-1) reduced splenic CD8− DCs to half of the control treatment in 1.5 days (Fig. 4). Similar reduction was observed at day 3 while it was restored at day7 (Fig. 4). In contrast, Notch signaling was essential for the development of MZ B cells (12) and no significant decrease of MZ B cells was detected at day 1.5 (Fig. 4). Notably, Annexin V-positive apoptotic CD8− DCs were increased in mice blocked all Notch ligands (3.03 ± 0.079% versus 2.37 ± 0.17% hamster IgG control mice, P = 0.0097).
Time course of CD8− DC reduction. Mice were treated with control hamster IgG (open columns) or a mixture of mAbs (HMD1-5, HMD4-2, HMJ1-29 and HMJ2-1; closed columns) once. Spleen cells were analyzed on indicated days. The percentages of CD11chigh/33D1+/CD8− (33D1+CD8− DC) among whole splenocytes and the percentage of MZ B cells (B220+/CD21hi/CD23lo/−) among total B cells (B220+) were analyzed by flow cytometry. Data are represented as the mean ± SD of three mice in each group. Similar results were obtained in two independent experiments. Data were analyzed statistically using unpaired Student's t-test. *P < 0.05 versus hamster IgG-treated control mice.
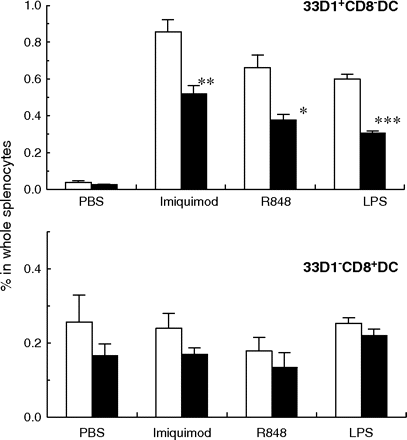
CD8− DC reduction is associated with decrease of IL-12 secretion from splenic DCs
To determine whether this decrease of CD8− DCs by blocking of Notch ligands was related to functional reduction, DC cytokine responses to toll-like receptors (TLR) activation was analyzed. Stimulation of splenocytes with TLR7 agonist, imiquimod or R848 or TLR4 agonist LPS induced IL-12 secretion from CD8− DCs in vitro (Fig. 5). IL-12-secreting CD8− DCs induced by these TLR agonists were decreased in splenocytes from mice treated with a mixture of antibodies against Notch ligands (HMD1-5, HMD4-2, HMJ1-29 and HMJ2-1) while IL-12-secreting CD8+ DCs were unchanged (Fig. 5).
Effects of anti-Notch ligand mAbs on IL-12 secretion from splenic DCs. Mice were treated with control hamster IgG (open columns) or a mixture of mAbs (HMD1-5, HMD4-2, HMJ1-29 and HMJ2-1; closed columns). Collagenase-treated splenocytes were cultured in the presence or absence of indicated TLR ligands. IL-12 production in DCs was detected by intracellular staining. The percentages of IL-12-secreting CD11chigh/33D1+/CD8− (33D1+CD8− DC) and CD11chigh/33D1−/CD8+ (33D1−CD8+ DC) cells among whole splenocytes were analyzed by flow cytometry. Data are represented as the mean ± SD of three mice in each group. Data were analyzed statistically using unpaired Student's t-test. *P < 0.05, **P < 0.01, ***P < 0.001 versus hamster IgG-treated control mice.
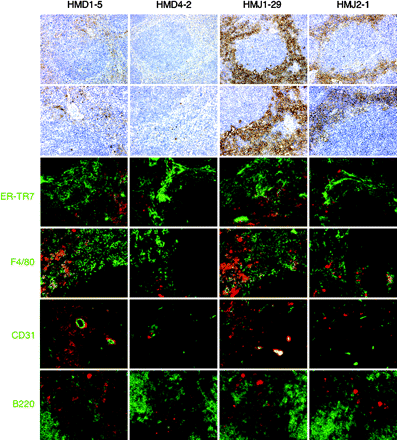
Localization of Notch ligands in the spleen
Finally, the location of Dll1, Dll4, Jagged1 and Jagged2 in the spleen was determined. Immunohistochemical analysis demonstrated that all these Notch ligands were expressed primarily in the red pulp area where CD8− DCs resided (Figs 2Band 6). ER-TR7+ reticular fibroblasts and F4/80+ macrophages localize mainly in the red pulp of the spleen. Accordingly, part of ER-TR7+ reticular fibroblasts expressed Dll1, Dll4, Jagged1 or Jagged2 (Fig. 6). Part of F4/80+ macrophages also expressed Dll1, Jagged1 or Jagged2 (Fig. 6). In addition, CD31+ vessels in the spleen expressed Dll1 or Jagged1 (Fig. 6). Though B cells were considered important in splenic DC homeostasis (13, 14), Notch ligands were not expressed on B cells (Fig. 6).
Immunohistological analysis for localization of Notch ligands in the spleen. Spleen sections were stained with HMD1-5, HMD4-2, HMJ1-29 or HMJ2-1 (brown or red) and ER-TR7 or mAbs against F4/80, CD31 or B220 (green). Essentially, no staining was observed with control hamster IgG (not shown). Original magnification: top panels, ×100; the others, ×200.
Discussion
Dll1 has been shown to be essential for the development of MZ B cells (4, 6). In this study, we found that Dll1 was also important for the maintenance of CD8− DCs, but Dll4, Jagged1 and Jagged2 could also participate in this process. Indeed, a similar extent of CD8− DC reduction was observed among Dll1-, Dll4-, Jagged1- and Jagged2-blocking mice and RBP-J CD11c-Cre+ cKO mice (3). Consistent with this notion, Jagged1 and Jagged2 were localized similar to Dll1. Therefore, it is likely that Jagged1 and Jagged2 as well as Dll1 could engage the Notch receptors on CD8− DCs to maintain this population. CD8+ DCs are reported to reside in the white pulp area (9) where Notch ligands were not expressed, which may explain why this subset was not regulated by Notch signaling. The functional role of Notch4 uniquely expressed on CD8+ DCs will be determined in our future study.
Notch2 was essential for the development of MZ B cells and expressed higher on MZ B and precursors of MZ B cells than on T1 B and T2/Fo B cells (12). Cell surface expression of Notch receptors suggest that Notch1, Notch2 and Notch3 could be responsible for the Notch signaling to maintain CD8− DCs in the spleen. The differential contribution of Jagged1 and Jagged2 to the homeostasis of CD8− DCs and MZ B cells may be explained by their difference in the expression of Notch receptors. Indeed, the expression of Notch1 and Notch2 on CD8− DCs was lower than that on B cells but Notch3 was expressed higher on CD8− DCs (12). Thus, Notch3 may be a key Notch receptor to maintain CD8− DCs via activation by Dll4, Jagged1 and Jagged2. Although Notch1 and Notch2 could be activated to maintain CD8− DCs especially in a Notch3-deficient condition, it is intriguing to investigate whether CD8− DCs are decreased in Notch3-deficient mice that are viable and fertile (15).
Splenic CD8− DCs are short-lived. Parabiosis experiments have estimated that the half-life of splenic CD8− DCs was ∼7 days (16). Inhibition of Notch ligands decreased splenic CD8− DCs earlier than its half-life at almost maximum level (1.5 days after mAbs treatment) in association with increased apoptosis of CD8− DCs and it restored quickly (at day 7). A recent study using DC-specific RBP-J KO mice has determined increased apoptosis of CD8− DCs in the spleen (3). These data indicate that Notch signaling contributes to survival of splenic CD8− DCs. Functional significance of CD8− DC reduction with the lack of Notch signaling has been demonstrated by the decrease of DC-mediated cytokine responses when injected with the TLR7 agonist imiquimod, which preferentially activates CD8− DCs (3). Blocking of Notch ligands also reduced TLR agonists-induced IL-12 secretion from CD8− DCs. MZ B cells are known to play roles in response to blood-borne pathogens and also in immune responses (17). Reduced MZ B cells through a defect of Notch signaling conferred high sensitivity to blood-borne bacteria, Staphlococcus aures, though it showed no obvious deficiency in the immune responses to thymus-dependent, thymus-independent type 1 and type 2 antigens (18).
Fringes are glycosyltransferases that modify the extracellular domain of Notch, enhancing its activation by Delta and inhibiting its activation by Jagged (19, 20). Therefore, the expression of Fringes in CD8− DCs may be different from that in MZ B cells, leading to the differential contribution of Delta and Jagged Notch ligands. Further studies are needed to address these possibilities.
Funding
Grants-In-Aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan (17016071).
Abbreviations
- cKO
conditional knockout
- Dll
Delta-like
- DC
dendritic cell
- ER
endoplasmic reticulum
- MZ
marginal zone
- TLR
toll-like receptors
References
Author notes
Transmitting editor: T. Watanabe